| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:01 UTC |
|---|
| HMDB ID | HMDB0000374 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 17-Hydroxyprogesterone |
|---|
| Description | 17-Hydroxyprogesterone also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. Formally it is a 17alpha-hydroxy steroid that is the 17alpha-hydroxy derivative of progesterone. 17-Hydroxyprogesterone is found in all vertebrates. It is a chemical intermediate in the biosynthesis of many endogenous steroids, including androgens, estrogens, glucocorticoids, mineralocorticoids and neurosteroids. In particular, 17-Hydroxyprogesterone serves as an intermediate in the biosynthesis of hydrocortisone and gonadal steroid hormones. It is derived from progesterone via the enzyme known as 17-hydroxylase, a cytochrome P450 enzyme also known as CYP17A1. It can also be biosynthesized from 17-hydroxypregnenolone via the enzyme 3beta-hydroxysteroid dehydrogenase/delta5-4 isomerase (PMID: 1955079 ). 17-OHP is an agonist of the progesterone receptor (PR). It is also an antagonist of the mineralocorticoid receptor (MR) as well as a partial agonist of the glucocorticoid receptor (GR). 17-Hydroxyprogesterone is a natural progestin and in pregnancy it increases in the third trimester primarily due to fetal adrenal production. 17-Hydroxyprogesterone is primarily produced in the adrenal glands and to some degree in the gonads, specifically the corpus luteum of the ovary. Normal levels are 3-90 ng/dl in children, and in women, 15-70 ng/dl prior to ovulation, and 35-290 ng/dl during the luteal phase. Measurements of levels of 17-hydroxyprogesterone are useful in the evaluation of patients with suspected congenital adrenal hyperplasia as the typical enzymes that are defective, namely 21-hydroxylase, lead to a build-up of 17-OHP. 17-OHP levels can also be used to measure contribution of progestational activity of the corpus luteum during pregnancy as progesterone but not 17-OHP is also contributed by the placenta. |
|---|
| Structure | [H][C@@]12CC[C@](O)(C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C InChI=1S/C21H30O3/c1-13(22)21(24)11-8-18-16-5-4-14-12-15(23)6-9-19(14,2)17(16)7-10-20(18,21)3/h12,16-18,24H,4-11H2,1-3H3/t16-,17+,18+,19+,20+,21+/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 17-Hydroxypregn-4-en-3,20-dione | ChEBI | | 17alpha-Hydroxy-4-pregnene-3,20-dione | ChEBI | | 17alpha-Hydroxy-progesterone | ChEBI | | delta(4)-Pregnene-17alpha-ol-3,20-dione | ChEBI | | Hidroxiprogesterona | ChEBI | | Hydroxyprogesterone | ChEBI | | Hydroxyprogesteronum | ChEBI | | Pregn-4-ene-3,20-dione-17-ol | ChEBI | | 17a-Hydroxy-4-pregnene-3,20-dione | Generator | | 17Α-hydroxy-4-pregnene-3,20-dione | Generator | | 17a-Hydroxy-progesterone | Generator | | 17Α-hydroxy-progesterone | Generator | | delta(4)-Pregnene-17a-ol-3,20-dione | Generator | | Δ(4)-pregnene-17α-ol-3,20-dione | Generator | | Δ(4)-pregnene-17a-ol-3,20-dione | HMDB | | 17-alpha-Hydroxyprogesterone | HMDB | | 17-Hydroxypregn-4-ene-3,20-dione | HMDB | | 17-OH Progesterone | HMDB | | 17-OHP | HMDB | | 17a-Hydroxypregn-4-ene-3,20-dione | HMDB | | 17a-Hydroxyprogesterone | HMDB | | 17alpha-Hydroxyprogesterone | HMDB | | D4-Pregnen-17a-ol-3,20-dione | HMDB | | Gestageno | HMDB | | Gestageno gador | HMDB | | Pregn-4-en-17a-ol-3,20-dione | HMDB | | Prodix | HMDB | | Prodox | HMDB | | 17 Hydroxyprogesterone | HMDB | | 17-Hydroxyprogesterone, (17 alpha)-isomer | HMDB | | 17 alpha Hydroxyprogesterone | HMDB | | 17 alpha-Hydroxyprogesterone | HMDB | | 17-Hydroxyprogesterone, (9 beta, 10 alpha)-isomer | HMDB |
|
|---|
| Chemical Formula | C21H30O3 |
|---|
| Average Molecular Weight | 330.4611 |
|---|
| Monoisotopic Molecular Weight | 330.219494826 |
|---|
| IUPAC Name | (1S,2R,10R,11S,14R,15S)-14-acetyl-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| Traditional Name | (1S,2R,10R,11S,14R,15S)-14-acetyl-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| CAS Registry Number | 68-96-2 |
|---|
| SMILES | [H][C@@]12CC[C@](O)(C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H30O3/c1-13(22)21(24)11-8-18-16-5-4-14-12-15(23)6-9-19(14,2)17(16)7-10-20(18,21)3/h12,16-18,24H,4-11H2,1-3H3/t16-,17+,18+,19+,20+,21+/m1/s1 |
|---|
| InChI Key | DBPWSSGDRRHUNT-CEGNMAFCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Alpha-hydroxy ketone
- Cyclic alcohol
- Tertiary alcohol
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organooxygen compound
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | - 17alpha-hydroxy steroid (CHEBI:17252 )
- Progestagens (C01176 )
- C21 steroids (gluco/mineralocorticoids, progestogins) and derivatives (LMST02030161 )
|
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 219 - 220 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.0065 mg/mL | Not Available | | LogP | 3.17 | HANSCH,C ET AL. (1995) |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 17-Hydroxyprogesterone,1TMS,isomer #1 | CC(=O)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | 3074.4 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,1TMS,isomer #2 | CC(=O)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 2919.7 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,1TMS,isomer #3 | C=C(O[Si](C)(C)C)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | 2959.5 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TMS,isomer #1 | CC(=O)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3001.1 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TMS,isomer #1 | CC(=O)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 2892.6 | Standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TMS,isomer #1 | CC(=O)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3226.4 | Standard polar | 33892256 | | 17-Hydroxyprogesterone,2TMS,isomer #2 | C=C(O[Si](C)(C)C)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | 3048.6 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TMS,isomer #2 | C=C(O[Si](C)(C)C)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | 2835.0 | Standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TMS,isomer #2 | C=C(O[Si](C)(C)C)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | 3293.2 | Standard polar | 33892256 | | 17-Hydroxyprogesterone,2TMS,isomer #3 | C=C(O[Si](C)(C)C)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 2909.1 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TMS,isomer #3 | C=C(O[Si](C)(C)C)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 2861.4 | Standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TMS,isomer #3 | C=C(O[Si](C)(C)C)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3342.1 | Standard polar | 33892256 | | 17-Hydroxyprogesterone,3TMS,isomer #1 | C=C(O[Si](C)(C)C)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 2983.4 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,3TMS,isomer #1 | C=C(O[Si](C)(C)C)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 2934.6 | Standard non polar | 33892256 | | 17-Hydroxyprogesterone,3TMS,isomer #1 | C=C(O[Si](C)(C)C)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3275.3 | Standard polar | 33892256 | | 17-Hydroxyprogesterone,1TBDMS,isomer #1 | CC(=O)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | 3308.3 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,1TBDMS,isomer #2 | CC(=O)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C(C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3187.4 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,1TBDMS,isomer #3 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | 3219.9 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TBDMS,isomer #1 | CC(=O)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C(C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3492.2 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TBDMS,isomer #1 | CC(=O)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C(C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3364.5 | Standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TBDMS,isomer #1 | CC(=O)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C(C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3440.2 | Standard polar | 33892256 | | 17-Hydroxyprogesterone,2TBDMS,isomer #2 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | 3551.5 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TBDMS,isomer #2 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | 3343.2 | Standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TBDMS,isomer #2 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | 3498.0 | Standard polar | 33892256 | | 17-Hydroxyprogesterone,2TBDMS,isomer #3 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C(C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3417.3 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TBDMS,isomer #3 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C(C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3306.9 | Standard non polar | 33892256 | | 17-Hydroxyprogesterone,2TBDMS,isomer #3 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C(C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3558.6 | Standard polar | 33892256 | | 17-Hydroxyprogesterone,3TBDMS,isomer #1 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C(C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3729.1 | Semi standard non polar | 33892256 | | 17-Hydroxyprogesterone,3TBDMS,isomer #1 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C(C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3566.3 | Standard non polar | 33892256 | | 17-Hydroxyprogesterone,3TBDMS,isomer #1 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CCC4=CC(O[Si](C)(C)C(C)(C)C)=CC[C@]4(C)[C@H]3CC[C@@]21C | 3515.6 | Standard polar | 33892256 |
|
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - 17-Hydroxyprogesterone GC-MS (2 MEOX; 1 TMS) | splash10-004l-4911000000-d40ba45835d1ee1b6c41 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 17-Hydroxyprogesterone EI-B (Non-derivatized) | splash10-002o-9651000000-b44110f9abcffa42ce35 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 17-Hydroxyprogesterone GC-MS (Non-derivatized) | splash10-004l-4911000000-d40ba45835d1ee1b6c41 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17-Hydroxyprogesterone GC-MS (Non-derivatized) - 70eV, Positive | splash10-0k9f-4595000000-89ccbf45dbf9f474bd2f | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17-Hydroxyprogesterone GC-MS (1 TMS) - 70eV, Positive | splash10-007c-3249000000-3570f5d248c0ee0716ee | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17-Hydroxyprogesterone GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17-Hydroxyprogesterone GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17-Hydroxyprogesterone GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17-Hydroxyprogesterone GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17-Hydroxyprogesterone GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17-Hydroxyprogesterone GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17-Hydroxyprogesterone GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-002f-9731000000-48d2266d41955ffa9ce4 | 2014-09-20 | Not Available | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - 17-Hydroxyprogesterone Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-001i-0009000000-a157e4b4aa18bdbc2531 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17-Hydroxyprogesterone Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-052b-5900000000-42494c0ef4b45cfa5cf4 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17-Hydroxyprogesterone Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-052b-9700000000-d8a942a3dfdb061bd5c3 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17-Hydroxyprogesterone EI-B (HITACHI M-80) , Positive-QTOF | splash10-002o-9651000000-b0c55e50343bf8ab3077 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17-Hydroxyprogesterone , positive-QTOF | splash10-0a4j-4930000000-c5056c0aac78a148ea92 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17-Hydroxyprogesterone 45V, Positive-QTOF | splash10-052b-5910000000-fcf792a46552ed6e0028 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17-Hydroxyprogesterone 60V, Positive-QTOF | splash10-052b-6900000000-6970a665800f041e9612 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17-Hydroxyprogesterone 30V, Positive-QTOF | splash10-0532-6966000000-f57233040f10efef4ba9 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17-Hydroxyprogesterone 15V, Positive-QTOF | splash10-001i-0009000000-0cff8a9a4549636b3f42 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17-Hydroxyprogesterone 75V, Positive-QTOF | splash10-0a4j-9800000000-3aaefadc8d2e4ad3a522 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17-Hydroxyprogesterone 90V, Positive-QTOF | splash10-0a6s-9600000000-17d56b65d46a46d9f653 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17-Hydroxyprogesterone 10V, Positive-QTOF | splash10-001i-0029000000-0af1b9a61aa5a9d514a4 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17-Hydroxyprogesterone 20V, Positive-QTOF | splash10-0lzi-0295000000-0510776f8fa0d9ddcb6a | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17-Hydroxyprogesterone 40V, Positive-QTOF | splash10-00li-3970000000-1655d52be304b45389ae | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17-Hydroxyprogesterone 10V, Negative-QTOF | splash10-004i-0009000000-bc250df2e599669d113e | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17-Hydroxyprogesterone 20V, Negative-QTOF | splash10-002r-0097000000-41db31593b4f58cd4bc4 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17-Hydroxyprogesterone 40V, Negative-QTOF | splash10-0bti-0092000000-464375e5c4a93d5d20b3 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17-Hydroxyprogesterone 10V, Positive-QTOF | splash10-01q9-0019000000-bf0f51dc62b5855371a3 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17-Hydroxyprogesterone 20V, Positive-QTOF | splash10-03di-0922000000-6997f50bd7502e7518f2 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17-Hydroxyprogesterone 40V, Positive-QTOF | splash10-0cdi-3910000000-051d81ed4aaf877bebaf | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17-Hydroxyprogesterone 10V, Negative-QTOF | splash10-004i-0039000000-8b733a2d2a661578ddbe | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17-Hydroxyprogesterone 20V, Negative-QTOF | splash10-004r-0049000000-1387b7ed039fc5504c87 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17-Hydroxyprogesterone 40V, Negative-QTOF | splash10-00kr-0091000000-dc494a2f8f424f99a574 | 2021-09-23 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, CDCl3, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CDCl3, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane (predicted from logP)
- Endoplasmic reticulum
|
|---|
| Biospecimen Locations | |
|---|
| Tissue Locations | - Adipose Tissue
- Adrenal Cortex
- Adrenal Gland
- Testis
|
|---|
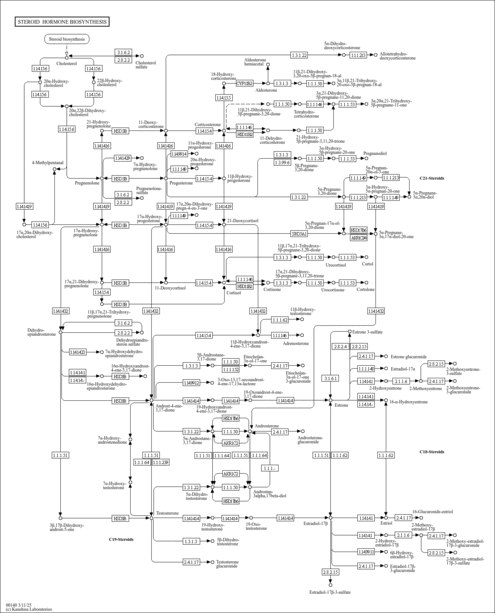
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.000212-0.00233 uM | Newborn (0-30 days old) | Female | Normal | | details | | Blood | Detected and Quantified | <0.0121 uM | Infant (0-1 year old) | Not Specified | Normal | | details | | Blood | Detected and Quantified | 0.00357 (0.00109-0.00539) uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.00454 +/- 0.00363 uM | Adult (>18 years old) | Female | Normal | | details | | Blood | Detected and Quantified | 0.011 (0.0003-0.023) uM | Infant (0-1 year old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.00151 +/- 0.000303 uM | Adult (>18 years old) | Female | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.000500-0.0200 uM | Not Specified | Not Specified | Normal | | details | | Blood | Detected and Quantified | <0.0450 uM | Newborn (0-30 days old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.000300-0.00510 uM | Infant (0-1 year old) | Not Specified | Normal | | details | | Blood | Detected and Quantified | <0.00500 uM | Not Specified | Both | Normal | | details | | Blood | Detected and Quantified | 0.000300-0.00950 uM | Children (1 - 13 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.00061 - 0.0136 uM | Adult (>18 years old) | Female | Normal | | details | | Blood | Detected and Quantified | 0.00061 - 0.00700 uM | Adult (>18 years old) | Male | Normal | | details | | Blood | Detected and Quantified | <0.00250 uM | Children (1-13 years old) | Both | Normal | | details | | Blood | Detected and Quantified | <0.00600 uM | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected and Quantified | <1.00 uM | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | <0.00015 umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.0011 (0-0.0019) umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.0013 +/- 0.00026 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | <0.000605 uM | Newborn (0-30 days old) | Female | Adrenal insufficiency, congenital, with 46,XY sex reversal, partial or complete | | details | | Blood | Detected and Quantified | 0.0832-0.339 uM | Newborn (0-30 days old) | Male | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | >0.303 uM | Children (1-13 years old) | Male | 21-hydroxylase deficiency | | details | | Blood | Detected and Quantified | >0.605 uM | Newborn (0-30 days old) | Male | 21-hydroxylase deficiency | | details | | Blood | Detected and Quantified | 484.17 uM | Adolescent (13-18 years old) | Female | 21-hydroxylase deficiency | | details | | Blood | Detected and Quantified | 6.99 uM | Newborn (0-30 days old) | Female | 21-hydroxylase deficiency | | details | | Blood | Detected and Quantified | 3873.39-12951.64 uM | Adult (>18 years old) | Female | 21-hydroxylase deficiency | | details | | Blood | Detected and Quantified | 1.522 uM | Infant (0-1 year old) | Both | Congenital Adrenal Hyperplasia, due to 21-Hydroxylase-Deficiency (CAH) | | details | | Blood | Detected and Quantified | 0.238 (0.0251-0.611) uM | Adult (>18 years old) | Both | 21-hydroxylase deficiency | | details | | Blood | Detected and Quantified | 0.0388-0.252 uM | Adult (>18 years old) | Female | 21-hydroxylase deficiency | | details | | Blood | Detected and Quantified | 0.00294 +/- 0.000900 uM | Newborn (0-30 days old) | Not Specified | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00170 +/- 0.00190 uM | Children (1-13 years old) | Not Specified | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.0057 uM | Adult (>18 years old) | Male | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.009 uM | Adolescent (13-18 years old) | Male | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00740-0.296 uM | Newborn (0-30 days old) | Both | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00450-0.184 uM | Children (1-13 years old) | Both | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00178 +/- 0.00144 uM | Infant (0-1 year old) | Not Specified | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00189 +/- 0.00100 uM | Children (1-13 years old) | Both | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00240 +/- 0.00220 uM | Adult (>18 years old) | Female | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00330 +/- 0.00180 uM | Adult (>18 years old) | Male | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.000908 uM | Adult (>18 years old) | Female | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | <0.000450 uM | Adult (>18 years old) | Female | Lipoid Adrenal Hyperplasia | | details | | Blood | Detected and Quantified | <0.000450 uM | Infant (0-1 year old) | Female | Lipoid Adrenal Hyperplasia | | details | | Blood | Detected and Quantified | <0.000450 uM | Adolescent (13-18 years old) | Female | Lipoid Adrenal Hyperplasia | | details | | Blood | Detected and Quantified | 0.0032 uM | Newborn (0-30 days old) | Female | Bartter Syndrome, Type 2, Antenatal | | details | | Blood | Detected and Quantified | 0.00130-0.0591 uM | Children (1-13 years old) | Both | 11-beta-hydroxylase deficiency | | details | | Blood | Detected and Quantified | 0.216-0.265 uM | Newborn (0-30 days old) | Female | 11-beta-hydroxylase deficiency | | details | | Blood | Detected and Quantified | 0.0147-0.0420 uM | Infant (0-1 year old) | Both | Antley-Bixler syndrome with genital anomalies and disordered steroidogenesis | | details | | Blood | Detected and Quantified | 0.152 (0.0022- 0.302) uM | Adult (>18 years old) | Both | Phenylketonuria | | details | | Blood | Detected and Quantified | 0.0014 (0.000030-0.0028) uM | Adult (>18 years old) | Both | 21-hydroxylase deficiency | | details | | Blood | Detected and Quantified | 0.016 (0.0090-0.024) uM | Children (1-13 years old) | Both | 21-Hydroxylase Deficiency (CYP21) | | details | | Blood | Detected and Quantified | <0.00300 uM | Newborn (0-30 days old) | Not Specified | 21-hydroxylase deficiency | | details | | Blood | Detected and Quantified | >0.300 uM | Newborn (0-30 days old) | Not Specified | 21-hydroxylase deficiency | | details | | Urine | Detected and Quantified | 0.0017 +/- 0.00034 umol/mmol creatinine | Adult (>18 years old) | Both | Systemic lupus erythematosus (SLE) | | details | | Urine | Detected and Quantified | 0.00065 +/- 0.00011 umol/mmol creatinine | Adult (>18 years old) | Both | Rheumatoid arthritis | | details | | Urine | Detected and Quantified | 0.00077 +/- 0.0002 umol/mmol creatinine | Adult (>18 years old) | Both | Rheumatoid arthritis | | details | | Urine | Detected and Quantified | 0.0022 +/- 0.00058 umol/mmol creatinine | Adult (>18 years old) | Both | Systemic lupus erythematosus (SLE) | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Phenylketonuria |
|---|
- Elvers LH, Loeber JG, Dhondt JL, Fukushi M, Hannon WH, Torresani T, Webster D: First ISNS Reference Preparation for Neonatal Screening for thyrotropin, phenylalanine and 17alpha-hydroxyprogesterone in blood spots. J Inherit Metab Dis. 2007 Aug;30(4):609. Epub 2007 Jun 14. [PubMed:17574536 ]
| | 21-Hydroxylase deficiency |
|---|
- Turcu AF, Rege J, Chomic R, Liu J, Nishimoto HK, Else T, Moraitis AG, Palapattu GS, Rainey WE, Auchus RJ: Profiles of 21-Carbon Steroids in 21-hydroxylase Deficiency. J Clin Endocrinol Metab. 2015 Jun;100(6):2283-90. doi: 10.1210/jc.2015-1023. Epub 2015 Apr 7. [PubMed:25850025 ]
- White PC, Speiser PW: Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000 Jun;21(3):245-91. doi: 10.1210/edrv.21.3.0398. [PubMed:10857554 ]
- Warinner SA, Zimmerman D, Thompson GB, Grant CS: Study of three patients with congenital adrenal hyperplasia treated by bilateral adrenalectomy. World J Surg. 2000 Nov;24(11):1347-52. [PubMed:11038205 ]
- Gmyrek GA, New MI, Sosa RE, Poppas DP: Bilateral laparoscopic adrenalectomy as a treatment for classic congenital adrenal hyperplasia attributable to 21-hydroxylase deficiency. Pediatrics. 2002 Feb;109(2):E28. [PubMed:11826238 ]
- Falhammar H, Wedell A, Nordenstrom A: Biochemical and genetic diagnosis of 21-hydroxylase deficiency. Endocrine. 2015 Nov;50(2):306-14. doi: 10.1007/s12020-015-0731-6. Epub 2015 Sep 4. [PubMed:26336836 ]
- G.Frauendienst-Egger, Friedrich K. Trefz (2017). MetaGene: Metabolic & Genetic Information Center (MIC: http://www.metagene.de). METAGENE consortium.
| | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency |
|---|
- Hattori N, Ishihara T, Moridera K, Hino M, Ikekubo K, Kurahachi H: A case of late-onset congenital adrenal hyperplasia due to partial 3 beta-hydroxysteroid dehydrogenase deficiency. Endocr J. 1993 Feb;40(1):107-9. [PubMed:7951484 ]
- Lutfallah C, Wang W, Mason JI, Chang YT, Haider A, Rich B, Castro-Magana M, Copeland KC, David R, Pang S: Newly proposed hormonal criteria via genotypic proof for type II 3beta-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 2002 Jun;87(6):2611-22. doi: 10.1210/jcem.87.6.8615. [PubMed:12050224 ]
- Guven A, Polat S: Testicular Adrenal Rest Tumor in Two Brothers with a Novel Mutation in the 3-Beta-Hydroxysteroid Dehydrogenase-2 Gene. J Clin Res Pediatr Endocrinol. 2017 Mar 1;9(1):85-90. doi: 10.4274/jcrpe.3306. Epub 2016 Jul 29. [PubMed:27476613 ]
| | 11-beta-Hydroxylase deficiency |
|---|
- Burren CP, Montalto J, Yong AB, Batch JA: CYP11 beta 1 (11-beta-hydroxylase) deficiency in congenital adrenal hyperplasia. J Paediatr Child Health. 1996 Oct;32(5):433-8. [PubMed:8933406 ]
| | Antley-Bixler syndrome with genital anomalies and disordered steroidogenesis |
|---|
- Fukami M, Hasegawa T, Horikawa R, Ohashi T, Nishimura G, Homma K, Ogata T: Cytochrome P450 oxidoreductase deficiency in three patients initially regarded as having 21-hydroxylase deficiency and/or aromatase deficiency: diagnostic value of urine steroid hormone analysis. Pediatr Res. 2006 Feb;59(2):276-80. doi: 10.1203/01.pdr.0000195825.31504.28. [PubMed:16439592 ]
| | Adrenal insufficiency, congenital, with 46,XY sex reversal, partial or complete |
|---|
- Kim CJ, Lin L, Huang N, Quigley CA, AvRuskin TW, Achermann JC, Miller WL: Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side chain cleavage enzyme, P450scc. J Clin Endocrinol Metab. 2008 Mar;93(3):696-702. doi: 10.1210/jc.2007-2330. Epub 2008 Jan 8. [PubMed:18182448 ]
| | Congenital Adrenal Hyperplasia, due to 21-Hydroxylase-Deficiency |
|---|
- Schwarz E, Liu A, Randall H, Haslip C, Keune F, Murray M, Longo N, Pasquali M: Use of steroid profiling by UPLC-MS/MS as a second tier test in newborn screening for congenital adrenal hyperplasia: the Utah experience. Pediatr Res. 2009 Aug;66(2):230-5. doi: 10.1203/PDR.0b013e3181aa3777. [PubMed:19390483 ]
| | Bartter Syndrome, Type 2, Antenatal |
|---|
- Chan WK, To KF, Tong JH, Law CW: Paradoxical hypertension and salt wasting in Type II Bartter syndrome. Clin Kidney J. 2012 Jun;5(3):217-20. doi: 10.1093/ckj/sfs026. Epub 2012 Mar 29. [PubMed:26069767 ]
| | Lipoid Congenital Adrenal Hyperplasia |
|---|
- Fujieda K, Tajima T, Nakae J, Sageshima S, Tachibana K, Suwa S, Sugawara T, Strauss JF 3rd: Spontaneous puberty in 46,XX subjects with congenital lipoid adrenal hyperplasia. Ovarian steroidogenesis is spared to some extent despite inactivating mutations in the steroidogenic acute regulatory protein (StAR) gene. J Clin Invest. 1997 Mar 15;99(6):1265-71. doi: 10.1172/JCI119284. [PubMed:9077535 ]
| | Rheumatoid arthritis |
|---|
- Straub RH, Weidler C, Demmel B, Herrmann M, Kees F, Schmidt M, Scholmerich J, Schedel J: Renal clearance and daily excretion of cortisol and adrenal androgens in patients with rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2004 Aug;63(8):961-8. [PubMed:15249323 ]
|
|
|---|
| Associated OMIM IDs | - 261600 (Phenylketonuria)
- 201910 (21-Hydroxylase deficiency)
- 201810 (Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency)
- 202010 (11-beta-Hydroxylase deficiency)
- 201750 (Antley-Bixler syndrome with genital anomalies and disordered steroidogenesis)
- 613743 (Adrenal insufficiency, congenital, with 46,XY sex reversal, partial or complete)
- 201910 (Congenital Adrenal Hyperplasia, due to 21-Hydroxylase-Deficiency)
- 241200 (Bartter Syndrome, Type 2, Antenatal)
- 201710 (Lipoid Congenital Adrenal Hyperplasia)
- 180300 (Rheumatoid arthritis)
|
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB021992 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 6002 |
|---|
| KEGG Compound ID | C01176 |
|---|
| BioCyc ID | 17-ALPHA-HYDROXYPROGESTERONE |
|---|
| BiGG ID | 36989 |
|---|
| Wikipedia Link | Hydroxyprogesterone |
|---|
| METLIN ID | 5363 |
|---|
| PubChem Compound | 6238 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 17252 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | 17AHPRGSTRN |
|---|
| MarkerDB ID | MDB00000154 |
|---|
| Good Scents ID | rw1368781 |
|---|
| References |
|---|
| Synthesis Reference | Schneider, Carlos; Silva, Mario; Zunza, Hilda; Becerra, Jose. Synthesis of 17.alpha.-hydroxyprogesterone from androstenedione. Boletin de la Sociedad Chilena de Quimica (1999), 44(2), 167-172. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - White PC, Tusie-Luna MT, New MI, Speiser PW: Mutations in steroid 21-hydroxylase (CYP21). Hum Mutat. 1994;3(4):373-8. [PubMed:8081391 ]
- Mellon SH, Miller WL: Extraadrenal steroid 21-hydroxylation is not mediated by P450c21. J Clin Invest. 1989 Nov;84(5):1497-502. [PubMed:2808702 ]
- Shackleton C, Malunowicz E: Apparent pregnene hydroxylation deficiency (APHD): seeking the parentage of an orphan metabolome. Steroids. 2003 Oct;68(9):707-17. [PubMed:14625002 ]
- Gibney MJ, Walsh M, Brennan L, Roche HM, German B, van Ommen B: Metabolomics in human nutrition: opportunities and challenges. Am J Clin Nutr. 2005 Sep;82(3):497-503. [PubMed:16155259 ]
- Sahin Y, Kelestimur F: 17-Hydroxyprogesterone responses to gonadotrophin-releasing hormone agonist buserelin and adrenocorticotrophin in polycystic ovary syndrome: investigation of adrenal and ovarian cytochrome P450c17alpha dysregulation. Hum Reprod. 1997 May;12(5):910-3. [PubMed:9194638 ]
- Kirchengast S, Hartmann B, Huber J: Serum levels of sex hormones, thyroid hormones, growth hormone, IGF I, and cortisol and their relations to body fat distribution in healthy women dependent on their menopausal status. Z Morphol Anthropol. 1996 Sep;81(2):223-34. [PubMed:9270338 ]
- Hampl R, Lachman M, Novak Z, Sulcova J, Starka L: Serum levels of steroid hormones in men with varicocele and oligospermia as compared to normozoospermic men. Exp Clin Endocrinol. 1992;100(3):117-9. [PubMed:1305061 ]
- Ibanez L, de Zegher F, Potau N: Anovulation after precocious pubarche: early markers and time course in adolescence. J Clin Endocrinol Metab. 1999 Aug;84(8):2691-5. [PubMed:10443661 ]
- Katagiri M, Kagawa N, Waterman MR: The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch Biochem Biophys. 1995 Mar 10;317(2):343-7. [PubMed:7893148 ]
- al Saedi S, Dean H, Dent W, Cronin C: Reference ranges for serum cortisol and 17-hydroxyprogesterone levels in preterm infants. J Pediatr. 1995 Jun;126(6):985-7. [PubMed:7776113 ]
- Gupta MK, Guryev OL, Auchus RJ: 5alpha-reduced C21 steroids are substrates for human cytochrome P450c17. Arch Biochem Biophys. 2003 Oct 15;418(2):151-60. [PubMed:14522586 ]
- Escobar-Morreale HF, San Millan JL, Smith RR, Sancho J, Witchel SF: The presence of the 21-hydroxylase deficiency carrier status in hirsute women: phenotype-genotype correlations. Fertil Steril. 1999 Oct;72(4):629-38. [PubMed:10521100 ]
- Wilson SC, Hodgins MB, Scott JS: Incomplete masculinization due to a deficiency of 17 beta-hydroxysteroid dehydrogenase: comparison of prepubertal and peripubertal siblings. Clin Endocrinol (Oxf). 1987 Apr;26(4):459-69. [PubMed:2820622 ]
- Toscano V, Sancesario G, Bianchi P, Cicardi C, Casilli D, Giacomini P: Cerebrospinal fluid estrone in pseudotumor cerebri: a change in cerebral steroid hormone metabolism? J Endocrinol Invest. 1991 Feb;14(2):81-6. [PubMed:2061573 ]
- Bolt RJ, Van Weissenbruch MM, Popp-Snijders C, Sweep FG, Lafeber HN, Delemarre-van de Waal HA: Maturity of the adrenal cortex in very preterm infants is related to gestational age. Pediatr Res. 2002 Sep;52(3):405-10. [PubMed:12193676 ]
- Lorence MC, Naville D, Graham-Lorence SE, Mack SO, Murry BA, Trant JM, Mason JI: 3 beta-hydroxysteroid dehydrogenase/delta 5----4-isomerase expression in rat and characterization of the testis isoform. Mol Cell Endocrinol. 1991 Sep;80(1-3):21-31. doi: 10.1016/0303-7207(91)90139-j. [PubMed:1955079 ]
|
|---|